What names are used to represent a single ionic or covalent compound?
The number of bonds that each element is able to form is usually equal to the number of unpaired electrons. In club to class a covalent bond, each element has to share ane unpaired electron.
Fig. 2.29 gives an instance of how to make a Lewis dot structure. First, make up one's mind how many atoms of each element are needed to satisfy the octet rule for each atom. In the formation of h2o, an oxygen atom has two unpaired electrons, and each hydrogen atom has one (Fig. 2.29 A). To fill its valence trounce, oxygen needs 2 additional electrons, and hydrogen needs ane. One oxygen atom can share its unpaired electrons with two hydrogen atoms, each of which demand only one additional electron. The single electrons match upwards to brand pairs (Fig. two.29 B). The oxygen atom forms two bonds, ane with each of 2 hydrogen atoms; therefore, the formula for water is H2O. When an electron, or dot, from one element is paired with an electron, or dot, from another element, this makes a bond, which is represented past a line (Fig. 2.29 C).
The number of bonds that an chemical element can form is determined by the number of electrons in its valence shell (Fig. two.29.1). Similarly, the number of electrons in the valence shell too determines ion formation. The octet rule applies for covalent bonding, with a total of eight electrons the most desirable number of unshared or shared electrons in the outer valence shell. For case, carbon has an atomic number of 6, with ii electrons in shell one and four electrons in trounce 2, its valence trounce (see Fig. 2.29.1). This means that carbon needs four electrons to achieve an octet. Carbon is represented with 4 unpaired electrons (see Fig. 2.29.1). If carbon can share 4 electrons with other atoms, its valence shell will be full.

Nearly elements involved in covalent bonding need eight electrons to have a consummate valence shell. Ane notable exception is hydrogen (H). Hydrogen can be considered to be in Group ane or Grouping 17 because it has properties similar to both groups. Hydrogen can participate in both ionic and covalent bonding. When participating in covalent bonding, hydrogen only needs two electrons to take a full valence shell. As information technology has only i electron to start with, information technology can simply make one bond.

Single Bonds
Hydrogen is shown in Fig two.28 with ane electron. In the formation of a covalent hydrogen molecule, therefore, each hydrogen cantlet forms a single bond, producing a molecule with the formula H2. A single bail is defined as one covalent bail, or two shared electrons, between two atoms. A molecule can take multiple unmarried bonds. For case, water, HiiO, has two single bonds, 1 betwixt each hydrogen cantlet and the oxygen atom (Fig. 2.29). Figure 2.thirty A has boosted examples of single bonds.
Double Bonds
Sometimes 2 covalent bonds are formed between 2 atoms by each cantlet sharing two electrons, for a total of iv shared electrons. For instance, in the formation of the oxygen molecule, each cantlet of oxygen forms ii bonds to the other oxygen atom, producing the molecule Oii. Similarly, in carbon dioxide (COii), ii double bonds are formed between the carbon and each of the two oxygen atoms (Fig. two.30 B).
Triple Bonds
In some cases, three covalent bonds tin can be formed between two atoms. The almost mutual gas in the atmosphere, nitrogen, is made of two nitrogen atoms bonded by a triple bail. Each nitrogen cantlet is able to share three electrons for a total of six shared electrons in the N2 molecule (Fig. 2.30 C).
Polyatomic Ions
In add-on to elemental ions, in that location are polyatomic ions. Polyatomic ions are ions that are made upwardly of 2 or more than atoms held together past covalent bonds. Polyatomic ions tin can join with other polyatomic ions or elemental ions to form ionic compounds.
Information technology is non easy to predict the name or charge of a polyatomic ion by looking at the formula. Polyatomic ions found in seawater are given in Table 2.10. Polyatomic ions bond with other ions in the aforementioned mode that elemental ions bond, with electrostatic forces caused past oppositely charged ions holding the ions together in an ionic compound bond. Charges must still be counterbalanced.
| Polyatomic Ion | Ion Proper noun |
|---|---|
| NH4 + | ammonium |
| CO3 2- | carbonate |
| HCOiii - | bicarbonate |
| NO2 - | nitrite |
| NOiii - | nitrate |
| OH- | hydroxide |
| PO4 three- | phosphate |
| HPOiv 2- | hydrogen phosphate |
| SiO3 2- | silicate |
| Then3 2- | sulfite |
| And then4 2- | sulfate |
| HSO3 - | bisulfite |
Fig. 2.31 shows how ionic compounds form from elemental ions and polyatomic ions. For example, in Fig. ii.31 A, it takes two K+ ions to balance the charge of one (SiO2)2- ion to form potassium silicate. In Figure 2.31 B, ammonium and nitrate ions have equal and opposite charges, so it takes one of each to grade ammonium nitrate.


P olyatomic ions can bond with monatomic ions or with other polyatomic ions to course compounds. In gild to class neutral compounds, the total charges must exist counterbalanced.
Comparison of Ionic and Covalent Bonds
A molecule or compound is made when two or more atoms grade a chemical bond that links them together. Equally nosotros take seen, there are two types of bonds: ionic bonds and covalent bonds. In an ionic bond, the atoms are spring together by the electrostatic forces in the attraction betwixt ions of opposite charge. Ionic bonds ordinarily occur between metal and nonmetal ions. For example, sodium (Na), a metal, and chloride (Cl), a nonmetal, form an ionic bond to brand NaCl. In a covalent bond, the atoms bond by sharing electrons. Covalent bonds usually occur betwixt nonmetals. For example, in water (H2O) each hydrogen (H) and oxygen (O) share a pair of electrons to make a molecule of two hydrogen atoms single bonded to a single oxygen cantlet.
In general, ionic bonds occur between elements that are far apart on the periodic table. Covalent bonds occur betwixt elements that are close together on the periodic table. Ionic compounds tend to be brittle in their solid form and have very loftier melting temperatures. Covalent compounds tend to be soft, and have relatively low melting and humid points. H2o, a liquid composed of covalently bonded molecules, can also be used as a test substance for other ionic and covalently compounds. Ionic compounds tend to dissolve in water (east.m., sodium chloride, NaCl); covalent compounds sometimes dissolve well in water (e.1000., hydrogen chloride, HCl), and sometimes do not (e.g., butane, C4H10). Properties of ionic and covalent compounds are listed in Table ii.11.
| Property | Ionic | Covalent |
|---|---|---|
| How bond is fabricated | Transfer of east- | Sharing of e- |
| Bail is between | Metals and nonmetals | Nonmetals |
| Position on periodic table | Opposite sides | Close together |
| Deliquesce in water? | Yeah | Varies |
| Consistency | Brittle | Soft |
| Melting temperature | Loftier | Depression |
The properties listed in Table ii.11 are exemplified by sodium chloride (NaCl) and chlorine gas (Cl2). Like other ionic compounds, sodium chloride (Fig. 2.32 A) contains a metal ion (sodium) and a nonmetal ion (chloride), is brittle, and has a high melting temperature. Chlorine gas (Fig. two.32 B) is similar to other covalent compounds in that information technology is a nonmetal and has a very low melting temperature.


Dissolving, Dissociating, and Diffusing
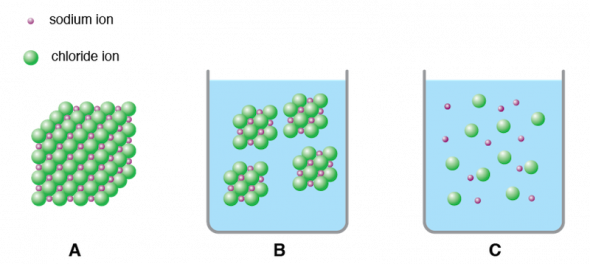
Ionic and covalent compounds too differ in what happens when they are placed in water, a common solvent. For example, when a crystal of sodium chloride is put into water, it may seem as though the crystal but disappears. Three things are really happening.
- A big crystal (Fig. 2.33 A) will dissolve, or break down into smaller and smaller pieces, until the pieces are too small to run into (Fig. 2.33 B).
- At the same fourth dimension, the ionic solid dissociates, or separates into its charged ions (Fig 2.33 C).
- Finally, the dissociated ions lengthened, or mix, throughout the water (Fig two.34).
Ionic compounds similar sodium chloride deliquesce, dissociate, and diffuse. Covalent compounds, like sugar and food coloring, can dissolve and diffuse, but they exercise non dissociate. Fig. 2.34, is a time series of drops of nutrient coloring diffusing in h2o. Without stirring, the food coloring will mix into the water through only the motility of the h2o and nutrient coloring molecules.

Dissociated sodium (Na+) and chloride (Cl-) ions in salt solutions tin course new salt crystals (NaCl) equally they get more than concentrated in the solution. As water evaporates, the salt solution becomes more and more concentrated. Somewhen, in that location is non enough water left to keep the sodium and chloride ions from interacting and joining together, so salt crystals grade. This occurs naturally in places like salt evaporation ponds (Fig. 2.35 A), in coastal tidepools, or in hot landlocked areas (Fig. 2.35 B). Salt crystals tin can also exist formed by evaporating seawater in a shallow dish, as in the Recovering Salts from Seawater Activity.


Source: https://manoa.hawaii.edu/exploringourfluidearth/chemical/chemistry-and-seawater/covalent-bonding
0 Response to "What names are used to represent a single ionic or covalent compound?"
Post a Comment